You are here
Instructions and Forms
A list of our currently active RFAs is available under Funding Opportunities.
Please also see our FAQ page for other information about applying to HEI.
Forms:
![]() Preliminary Application Form
Preliminary Application Form
![]() Full Application Form
Full Application Form
![]() Biosketch Form
Biosketch Form
![]() Application Instructions (a pdf of the text on this page)
Application Instructions (a pdf of the text on this page)
Submission:
- Preliminary Applications: All Preliminary application materials should be combined into a single PDF (max size 10MB) named “PI lastname firstname RFA xx-x Preliminary Application.” Submit online at: https://www.surveymonkey.com/r/X79G9Q5.
- Full Applications: Submit to funding@healtheffects.org.
Application Instructions:
Part 1: Preliminary Applications
Part 2: Full Applications
Related Policies:
HEI Project Negotiations and Investigator Commitments
Quality Assurance and Quality Control Procedures for HEI Studies
Data Access Policy
HEI's Conflict of Interest Policy
PART 1: PRELIMINARY APPLICATIONS
Applicants should use the HEI Preliminary Application Form (available above) that requests the following information: title, plain language summary, project description including the study aims, design and methods, and anticipated results, project plan, community engagement plan where applicable, qualifications of investigators, and biographical sketches.
The preliminary application must not exceed 5 pages (11-point font size and 1-inch margins, single-spaced), including the cover material, but excluding references, biosketches, and supporting letters, where applicable. Longer applications will be rejected. The application forms should be converted to PDF format before submitting.
PLAIN LANGUAGE SUMMARY
The plain language summary should indicate the purpose of the study, population(s) to be studied, and datasets and approaches to be used (max 150 words).
STUDY DESCRIPTION
General
The study description should include the following: scientific background and rationale, hypothesis and study aims, preliminary or relevant data from previous studies, study design and methods, statistical analyses, and anticipated results. The project plan should discuss the novelty of the proposed approach and how the study addresses the RFA objectives and satisfies the criteria listed in the RFA under “Evaluation Process for Full Applications.”
Community Engagement
Proposals where community engagement is indicated should describe community stakeholders that the investigators plan to engage, the general approach to engagement, and integration of community stakeholders into the proposed research (an additional ½ page). Specify how the community engagement will benefit the research and community members. Applications that require high levels of community engagement, such as sampling within a community or community partnerships, require a Letter of Collaboration to be sent with the Preliminary Application.
Qualifications of the Investigator(s)
Explain how the team is uniquely positioned to deliver results and why the team is the best choice and has the resources to meet the objectives stated in the RFA. The preliminary application should specify the expertise and experience of anticipated collaborators and briefly describe how their expertise would contribute to designing and conducting the study, analyzing the data, and interpreting study findings. Highlight experience with relevant study design and data collection methods (e.g., field work) and community engagement. Please note that the Principal Investigator may not be a graduate student or postdoctoral researcher.
BIOGRAPHICAL SKETCH
Include biographical sketches of the principal investigator and key co-investigators. Applicants can use the HEI Biosketch form (available above) or another format as long as each biosketch is no longer than two pages.
SUPPORTING LETTERS
Include letters of support as indicated in the RFA at the preliminary application stage.
ROSENBLITH APPLICATIONS, ONLY
Rosenblith Award applicants should include only their own biosketch. Co-investigators are not allowed under the Rosenblith RFA, but junior personnel are allowed. Mentors should be listed on the form. A cover letter, mentor biosketches, and letters of support are NOT needed at this stage.
PART 2: FULL APPLICATIONS
GENERAL INFORMATION
Full applications (by invitation only) must be submitted on the HEI Application for Research Agreement (Forms F-1 to F-12). Applications should be typed single-spaced, within the margin limitations indicated on the forms (1-inch minimum), and use a minimum font size of 11 pt.
HEI and its funded institutions are subject to the Office of Management and Budget and EPA accounting regulations. Please provide a DUNS number for your institution.
ROSENBLITH APPLICATIONS ONLY
COVER LETTER
A cover letter (maximum 2 pages) from the applicant that describes their interest in the award and how this project fits with their career goals, including information concerning their long-term career plans and how the HEI award would contribute to these plans. The letter should also include how this award will help the applicant on a pathway to independence.
FACE PAGE (FORM F-1)
This form must be signed and dated by a legal representative and the study principal investigator.
TABLE OF CONTENTS (FORM F-2)
Finalize the table of contents and add page numbers once the application has been completed.
ABSTRACT OF THE PROJECT PLAN (FORM F-3)
Concisely describe the project’s specific aims, methods, and long-term research objectives. Refer to the scientific disciplines involved and the relationship of the project to the objectives of HEI and the Request for Applications. The abstract should be self-contained so that it can serve as a succinct and accurate description of the application when separated from it. Do not exceed one page.
BUDGET (FORMS F-4 AND F-5)
Cost or Pricing Data: Provide adequate data and analysis to assure HEI that the proposed costs are necessary and reasonable and that adequate accounting procedures will be used. Please refer to the RFA for specific instructions on projected start date and budget caps.
PERSONNEL
List the names and positions of all applicant organization personnel involved in the project, both professional and nonprofessional, whether or not salaries are requested. Note that under the Walter A. Rosenblith New Investigator Award, senior personnel and mentors cannot be included for salary support. Estimate the percentage of time or effort or hours per week on the project for professional personnel in relation to the total professional activity commitment to the applicant organization, and estimate the hours per week on the project for nonprofessional personnel. List the dollar amounts separately for each individual for salary and fringe benefits. Fringe benefits may be requested to the extent that they are treated consistently by the applying organization as a direct cost to all sponsoring agencies.
The amount to be reimbursed to each individual, when added to their compensation for all other full-time duties, should not exceed the individual’s base salary. In computing estimated salary changes, an individual’s base salary represents the total authorized annual compensation that an applicant organization would be prepared to pay for a specific work period whether an individual’s time is spent on sponsored research, teaching, or other activities. The base salary for the purposes of computing charges to an HEI Research Agreement excludes income that an individual may be permitted to earn outside of full-time duties to the applicant organization.
Where appropriate, indicate whether the amounts requested for the principal investigator and other professional personnel are for summer salaries or academic-year salaries and indicate the formulas for calculating summer salaries.
Indicate whether current rates or escalated rates are used. If escalation is included, state the degree (percent) and methodology (e.g., annual flat rate applied to base rate as of a specific date or a mid-point rate for the period of performance).
CONSULTANT COSTS
Consultant service should be explained by indicating the specific area in which such service is to be used. Identify the contemplated consultants. State the number of days of such services estimated to be required and the consultant’s quoted rate per day and indicate the number of hours per day in which work will be performed. The maximum consultant rate is $650/8-hr day. HEI’s participation in consultant costs is subject to limits set by federal regulations. (See also Additional Submissions below).
SUPPLIES AND OTHER EXPENSES
All supplies and other expenses should be itemized in sufficient detail to allow reviewers to understand the major categories of expenditures (i.e., glassware, media, chemicals, animal purchase and housing, as well as publication costs, page charges, and books, listed by category and unit cost). Itemize and justify such items as patient compensation, travel, per diem costs, rentals, leases, and computer costs. Unusually expensive items for special processes should be separately identified by quantity and price and the use or application thoroughly explained in the project plan. Each individual expense item must be categorized as supplies or other expenses according to the practices of the accounting office of your institution. Items that cost more than $5,000 should be listed under equipment (see below).
The costs of construction per se are not permissible charges. If the costs of essential alterations of facilities, including repairs, painting, removal or installation of partitions, shielding, or air conditioning, are requested, itemize them by category and justify them fully. When applicable, indicate the square footage involved, giving the basis for the costs, such as an architect’s or applicant’s detailed estimate. When possible, submit a line drawing of the alterations being proposed.
TRAVEL EXPENSES
Limit travel to one scientific meeting per year. Do not include the travel to the HEI Annual Conference within the budget because HEI will cover these costs directly. If travel is required for other purposes, such as meetings with collaborators, indicate the estimated number of trips, destination, reason for travel, and cost. Identify and support any other special transportation costs attributable to the performance of this project. HEI pays for foreign travel only if it is approved in advance of the trip.
INDIRECT COSTS
Indirect costs are limited to a maximum of 30% of direct costs excluding equipment charges and subcontracts. In addition, indirect costs cannot be greater than the government-negotiated rate for your institution (e.g., if the approved rate is 25%). Expenses normally included in the calculation of the indirect cost rate may not be itemized as direct expenses.
The HEI Board of Directors has approved a very limited exception to this cap on indirect costs for organizations that can meet both of the following conditions: (1) the research institution provides a unique capability for a project essential to HEI’s mission, and (2) the institution is prohibited by the U.S. Government from accepting less than full cost recovery.
EQUIPMENT
Provide an itemization and justification of all equipment to be purchased or fabricated for use in this study. Please note that HEI reimburses institutions only for those equipment items explicitly listed in the Approved Budget or subsequently authorized in writing by HEI’s Director of Science or Director of Finance & Administration. The equipment budget is not subject to indirect cost charges.
SUBCONTRACTS
Itemize and enter a total for these costs. Describe and justify all appropriate costs for services purchased for, or associated with, third parties, including applicable indirect costs. These costs may include consortium agreements or formalized collaborative agreements. Indirect costs for subcontracts are also subject to HEI’s 30% cap (see above). Develop separate budgets for the initial and future budget periods for each organization involved in consortium arrangements or formalized collaborative agreements and submit them using the appropriate budget form (F-4b and F-5b). The principal investigator’s institution may charge indirect costs to the first $25,000 of each subcontract.
FINAL REPORT PREPARATION (Final study year only)
The final report is due at the end of the final contract period at the 12-month time point. Investigators must budget funds and an appropriate percentage effort for writing the report in the final year of the study. These funds are included as part of the maximum allocated budget as specified in the research solicitation. Please refer to the Final Report section in HEI Investigator Commitments for details.
Investigators should also be aware that after the study has been completed and the report submitted, additional time and effort is expected to address revisions requested by the Review Committee and to answer editorial queries.
The final contract period is therefore 18 months to accommodate completing the research, preparing the final report, and submitting the report no later than the end of the 12th month with an additional 6 months provided for revisions.
Contingent upon submission of the Final Investigator’s Report to HEI at the end of the 12th month of the final contract period, HEI provides up to an additional 6 months and up to $20,000 beyond the maximum allocated research budget to cover appropriate personnel effort revising an already submitted report. Include this projected cost under “Report Revision and Editing.” These funds may not cover report preparation. Unspent funds may not be reallocated.
BUDGET JUSTIFICATION
a. Personnel and Consultants. For the total budget and subcontracts, briefly describe the specific functions of the personnel and consultants. For each year, justify any cost for which the need might not be obvious, such as equipment, foreign travel, alterations and renovations, and contractual or third-party costs. For future years, justify any significant increases in any category. If a recurring annual increase in personnel costs is anticipated, give percentage. Note that an Institutional Cost Rate Agreement should be submitted once the project has been approved for funding. Include justification for the funds allocated for report revision and editing.
b. Community Engagement. Describe the costs associated with community engagement, including developing partnerships, holding community and stakeholder meetings, and developing print and electronic materials to communicate information relevant to study activities and results.
c. Preparation of Quality Assurance Project Plan. Before research commences, and in the first year of funding, Investigators must prepare a Quality Assurance Project Plan. All costs associated with preparation of the Plan should be indicated here, including costs of a Quality Assurance Manager (see QA/QC policy).
d. Data Accessibility. Specify the costs associated with retaining the data and making it publicly accessible in accordance with HEI’s Policy on Data Management, Preservation, and Access.
PROJECT PLAN (FORM F-6)
The Project Plan should include all the sections listed below. Include sufficient information in the Project Plan and in any appendix to facilitate an effective review. Be specific and informative and avoid redundancies. Sections A, B, and C together should total no more than four single-spaced pages. The Institute reserves the right not to consider proposals that exceed this limit. Appendices may be provided as supplementary information, but the review will be based mainly on the information provided in the Project Plan. Section D should be concise but adequately detailed to permit critical evaluation. Sections D and E combined should not exceed 15 pages (excluding references). Please use an 11-point font size or larger and 1-inch margins.
A. Specific Objectives
State concisely and realistically what the research described in this application is intended to accomplish or what hypothesis is to be tested.
B. Anticipated Results and Significance
Briefly sketch the background to the present proposal, critically evaluate existing knowledge, and specifically identify the gaps that the project is intended to fill. State concisely the importance of the research described in this application by relating the specific aims to the stated objectives of HEI and explain the regulatory significance.
C. Related Previous Studies
Provide an account of, and references to, the principal investigator’s previous studies pertinent to the application and any other information, including preliminary findings, that will help to establish the experience and competency of the investigator to pursue the proposed project. The appendix can be used for published references or details of available pilot studies.
D. Experimental Plan and Methods
Discuss in detail the experimental design and the procedures to be used to accomplish the specific aims of the project.
Define your study sample (such as cell type, animal strain, or subject population) and explain the rationale for choosing it. If the study involves human participants, describe how they will be selected and the informed consent procedure. (See Additional Submissions, Form F-11 below). If the study involves collection of environmental samples, describe the sampling frequency and duration.
HEI is committed to research that can lead to a better understanding of the health responses of all members of the general population, particularly the most sensitive. Accordingly, consider the composition of the study population, including gender, racial/ethnic composition, and other aspects that might affect the response, and provide a rationale for the choice of composition.
Provide sufficient details of the experimental design and study protocol so that it can be understood clearly by the reviewers. Applicants should provide details of exposure systems for specific pollutants (and the rationale for their selection), randomization procedures, methods used for any blinding of observations, and the proposed number of observations (including the number of animals or participants and exposure groups), preferably with a power calculation. Describe any new methodology and its advantage over existing methodologies. For in vitro and in vivo exposure studies, methods to generate, monitor, and analyze exposure concentrations and composition should be described in detail. HEI is not enthusiastic about funding studies with older engines and fuels or Standard Reference Materials samples; however, they could serve as positive controls for the proposed experimental treatment.
Discuss the potential difficulties and limitations of the proposed procedures and alternative approaches to achieve the aims.
Where appropriate, describe the procedures to be used to ensure that the quality of the data is adequate in view of the objectives of the study (see Quality Assurance and Quality Control in HEI Investigator Commitments). However, detailed QA information should not be submitted with the original application but will be requested for successfully funded studies that meet the above criteria.
E. Statistical Design, Analysis Plans, and Access to Data
Provide calculation of statistical power and a justification of the proposed numbers of animals/participants/samples. Include a description of the statistical methods to be used for analysis and interpretation of the data. Describe the proposed statistical procedures with sufficient detail to allow evaluation by a (bio)statistical reviewer. It should be evident that a statistician has reviewed the study design and data analysis plan. HEI recommends including power calculations where possible. Please note that in addition to reviews by experts in the subject matter, HEI often asks statisticians to review the statistical design of studies.
Investigators should include a plan for data sharing and data accessibility at the end of the study. Please see HEI’s Policy on Data Access.
F. Research Translation and Dissemination
The Research Translation and Dissemination Plan describes approaches for providing education and outreach to various sectors, such as community members and policymakers. The plan should outline the team members and other collaborators involved in implementing the plan, and how and to whom results will be communicated, including dissemination beyond academic presentations and publications where possible. The plan should include the insights (e.g., intended and unintended effects) and decision-making applications that the findings can provide as well as the potential limitations, uncertainties, and the factors (e.g., built, natural, socioeconomic, and political) captured or not captured in the research. For applications with community engagement, please cross reference that plan (Form F-7).
G. Milestones and Timeline
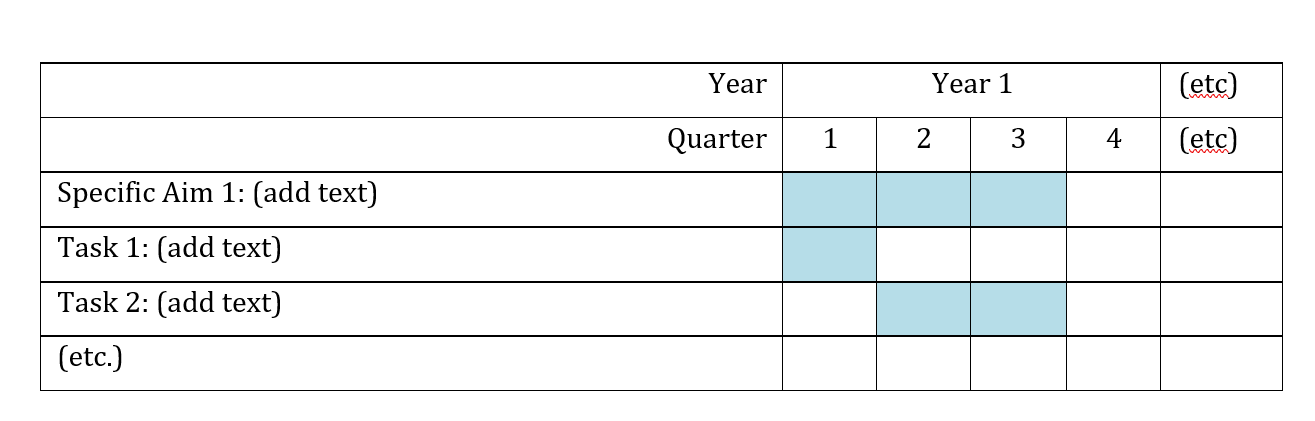
Please include a list of milestones to be met during the project with a timeline. Please use this template and fill in the cells to indicate when a specific aim or task will be ongoing and completed. If a study is recommended for funding, HEI will request a preliminary QA/QC plan to be submitted to HEI on contract initiation. A final approved plan is required within 2 months of the contract start date and before study initiation (i.e., data collection). We recommend that investigators allocate 2 months at the start of the study period to develop and finalize their Quality Assurance/Quality Control Plan, about 3 months at the end of the study to write a final report, and the final 6 months for revising the final report in response to Review Committee and editorial comments. Please note that the final contract period lasts 18 months, which includes research time (9 months), report writing (3 months), and report revisions (final 6 months). The report is due at the end of the 12th month of the final contract period.
Milestone Chart:
H. Literature Cited
References in the text should consist of the author and year. Provide complete citations in alphabetical order at the end of the Project Plan.
COMMUNITY ENGAGEMENT PLAN (FORM F-7 — refer to RFA to determine if applicable).
The Community Engagement Plan should describe engagement with community groups and other end users of the work that have an interest in the proposed research. Community engagement has various meanings and can be conducted through a variety of actions to broaden the impact of studies while simultaneously benefiting the communities involved. The level and type of community engagement should be appropriate for the proposed study. Cross reference your Project Plan (Form F-6) to indicate when and how you will engage with communities, explain how the engagement will contribute to achieving research objectives, and ensure that your budget (forms F-4 and F-5) reflects proposed community engagement activities. The community engagement plan may not exceed two pages.
The plan should provide the following information:
a. Objectives for engagement. Describe how engagement will benefit both the research and community members. Include anticipated outcomes of engagement.
b. Anticipated community groups and other end users. Describe the anticipated community and end users that the applicants plan to engage or partner and the approach that will be used to identify them. Include the broader context (e.g., historical or social) of efforts by the community or others to address the problem or issue, and how the proposed research will fit into these efforts, including whether the researchers are responding to a community-initiated idea.
a. Please also include a letter of collaboration as part of Form F-11 from the collaborators who have an active role in the research. In this letter, the collaborator should describe how they will benefit from and support the study and any past, ongoing, or hoped-for future collaborations with the academic researchers.
c. Approach to engage community groups and other end users. Describe the strategies and the specific roles of collaborators throughout the study period to conduct constructive community engagement and foster relationships during research. Describe how or if the anticipated community or other end users will be involved in designing, implementing, or communicating the research. Include information on how community members and groups who might play a more active role in the research will be compensated for their time and expenses. Explain the strategy for lowering barriers to participation (e.g., enhance cultural competency, provide multiple engagement opportunities, or provide childcare).
d. Approach for communication of study designs and results. Describe the communication products that you plan to produce (e.g., posters, workshops, or manuscripts), intended audiences of those products, and approach to ensuring that research translation and communication of study design, progress, and results occur equitably and through culturally appropriate means.
e. Measures of success. Describe how you will measure progress of the desired outcomes for community engagement based on both outputs and outcomes. Examples of output measures could include the number of workshops and open houses organized, the number of community members participating in meetings, the diversity and range of interests represented at meetings, and the number of communication activities conducted. Other outcome measures would be obtained through surveys provided to stakeholders and the public that capture responses to engagement-related questions and that track changes over time. If the research consists of a formal partnership between a research institution and community-based organization (see Box 2 for definition), describe mechanisms for feedback and improvement over the course of the research.
f. Research team member expertise. Describe the team members who have the expertise and responsibility to implement the community engagement plan, including previous experience with community engagement in environmental health research and if those members have received training relevant to equity and equitable relationships with community groups.
Box 1. Community Engagement
The National Institutes of Health defined community engagement as “…the process of working collaboratively with and through groups of people affiliated by geographic proximity, special interest, or similar situations to address issues affecting the wellbeing of those people.” Community engagement can be implemented on a continuum of community involvement in the research, ranging from outreach to shared leadership (Clinical Translational Science Awards Consortium, Community Engagement Key Function Committee Task Force on the Principles of Community Engagement 2011).
The National Science Foundation (2022) provided the following examples of community engagement activities:
• Holding roundtables and community meetings as well as conducting surveys to understand community member needs and concerns, and to develop and refine the research.
• Incorporating communities into processes for identifying key issues, planning and implementing projects, decision making, and evaluating outcomes.
• Providing data, facilities, resources, and expertise instrumental to the project.
• Conceiving of and supporting research demonstrations, experimentation, proofs of concept, or pilot projects.
• Participating in “living labs” where technological and social advances are staged iteratively through pilot studies in communities.
• Assisting in planning and implementation of evaluations of proposed research, including helping to define or create metrics and support data collection and/or interpretation within the community context.
• Public participation and engagement in research and data collection, including crowdsourcing.
Communities or end users can include some or all the following: local residents, neighborhood or community groups, nonprofit or philanthropic organizations, businesses, and municipal organizations such as libraries, public works departments, health and social services agencies, and schools, as well as regional end users, such as local, county, and state governments and departments and regional cooperative initiatives (adapted from National Science Foundation, 2022).
Sources:
Clinical Translational Science Awards Consortium, Community Engagement Key Function Committee Task Force on the Principles of Community Engagement. 2011. Principles of Community Engagement (2nd edition). NIH Publication no. 11–7782. Washington, DC: Department of Health and Human Services.
National Science Foundation Smart and Connected Communities (NSF 22-529) Program Webinar. 2022. Available at: https://new.nsf.gov/events/smart-connected-communities-nsf-22-529-program.
Box 2: Community-Based Organization Definition
HEI has adopted U.S. Environmental Protection Agency’s definition of a “community-based non-profit organization” (CBO): a public or private nonprofit organization that supports and/or represents a community and/or certain populations within a community through engagement, education, and other related services provided to individual community residents and community stakeholders. A “community” can be characterized by a particular geographic area and/or by the relationships among members with similar interests and can be characterized as part of a broader national or regional community where organizations can be focused on the needs of urban, rural, and/or tribal areas, farmworkers, displaced workers, children with high levels of lead, people with asthma, subsistence fishers, and other similar groups (U.S. EPA 2023).
The expectation is that the CBO should be driven by community residents in core aspects of its existence, such as:
• The majority of the governing body and staff consists of local residents.
• The main operating offices are in the community.
• Priority issue areas are identified and defined by residents.
• Solutions to address priority issues are developed with residents.
• Program design, implementation, and evaluation components have residents intimately involved in leadership positions. (National Community-Based Organization Network 2023)
Sources:
U.S. Environmental Protection Agency. 2023. The Environmental Justice Thriving Communities Grantmaking Program (EJ TCGM). https://www.epa.gov/environmentaljustice/environmental-justice-thriving-communities-grantmaking-program. Accessed November 2, 2023.
National Community-Based Organization Network. What is a CBO? 2004. https://sph.umich.edu/ncbon/about/whatis.html. Accessed November 2, 2023
OTHER SUPPORT (FORM F-8)
Describe current and pending grants or contracts — whether from public or private sources — from which each of the key investigators included in the proposed project are now drawing or anticipate drawing support, including any in-kind contributions. Identify project by title, agency, or organization supporting such work, and the total level of financial support given for the project, the percentage of time (or calendar months) spent on each project, and the (projected) start and end dates. Briefly describe the contents of each. If any of these overlap, duplicate, or are being replaced or supplemented by the present application, justify and delineate the nature and extent of the scientific and budgetary overlaps or boundaries.
RESOURCES AND ENVIRONMENT (FORM F-9)
A. Facilities
Describe all the facilities to be used and indicate their capacities, pertinent capabilities, relative proximity and extent of availability to the project. Using continuation pages (if necessary) include a description of the nature of any collaboration with other organizations and provide further information in the Project Plan (Form F-6).
B. Major Equipment
List the most important equipment items available for this project, noting the location and pertinent capabilities of each.
C. Sponsor Participation
If yes, on a separate page identify and explain role of any individuals employed by HEI sponsors (U.S. Environmental Protection Agency, industry, or foundations) who are involved with any aspect of the project. Also, list any resources provided by sponsors (such as facilities or animals).
BIOGRAPHICAL SKETCHES (FORM F-10)
Provide information on the education and research and professional experience of professional personnel and consultants beginning with the Principal Investigator. Do not exceed 2 pages per individual.
ADDITIONAL SUBMISSIONS (FORM F-11)
Human Participants (if applicable)
Safeguarding the rights and welfare of human participants in projects supported by EPA grants is the responsibility of the institution, which receives or is accountable to EPA for the funds awarded for the support of the project. The EPA regulations require applicant institutions to comply with the Department of Health and Human Services (DHHS) guidelines for human participants and additional requirements specified by the EPA. HEI is responsible for ensuring that these guidelines are followed by all institutions and investigators who receive HEI funds.
Applicants who request research funds from HEI are asked to indicate on the Title Page (Form F-1, Item 6), whether human subjects or derived materials will be used during their study. If Item 6 of the application has been marked “YES,” applicants should briefly describe the nature of human participation (or use of human data) and indicate whether IRB assurance is already in place or will be requested. Applicants based outside the United States should briefly describe the process for obtaining permission from their Ethics Board or equivalent body that provides such approvals.
Investigators who are selected for funding will need to submit the following to HEI before the study starts: OMB form No. 0990-0263, application to IRB (or equivalent ethics board) and relevant documents, approval from IRB or a statement from the IRB that the study is exempt, and approved informed consent documents, if applicable.
Laboratory Animals (if applicable)
The applicant shall provide with the application written assurance that any use of laboratory animals will comply with the provisions of the Animal Welfare Act (7 U.S.C. S 2131 et. seq.) and the guidelines set forth in the Guide for the Care and Use of Laboratory Animals. These documents are available from the Office for the Protection from Research Risks, National Institutes of Health, Bethesda, MD 20892. When laboratory animals are to be used in the proposed studies, state the species, strains, ages, and numbers of the animals involved and the methods to be used to comply with the above-mentioned guidelines. If approval from the Institutional Animal Care and Use Committee has been obtained, the approval letter should be included with the application. Investigators are also encouraged to read the Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines (see http://www.nc3rs.org.uk/page.asp?id=1357). Although these guidelines pertain to reporting of research, HEI urges investigators to plan anim
Recombinant DNA (if applicable)
Applicants proposing work with recombinant DNA should adhere to the current NIH Guidelines for Research Involving Recombinant DNA Molecules.
Quality Assurance (QA)/Quality Control (QC)
All applicants should provide a high-level, half-page summary of the measures in place to ensure the quality of the data collection procedures, data processing procedures and analyses, and data and records management. Please list the standard operating procedures that exist or will be developed for the methods laid out in the proposal.
Detailed QA/QC information should not be submitted with the original application but will be requested for successfully funded studies. If a study is recommended for funding, HEI will request a preliminary QA/QC plan to be submitted to HEI on contract initiation. A final approved plan is required within 2 months of the contract start date and before study initiation (i.e., data collection). HEI typically contracts an external QA auditor for all studies. See HEI Investigator Commitments and our QA/QC procedures page for more details.
Sponsor Participation
If “YES” has been marked under sponsor participation (i.e., any of the organizations funding HEI) on page F-8 of the application form (Other Support, Form F-8), please explain on a separate sheet the nature of sponsor participation. Identify and explain the role of any individual employed by EPA or industry sponsors of HEI (see www.healtheffects.org/about/sponsors) who is involved with any aspect of the proposed study. Also, list any resources provided by sponsors, including animals, equipment, and facilities. Please note that employees of organizations funding HEI cannot receive funds from HEI for salary or any other costs.
Previous HEI Funding
All principal investigators (and co-PI, if applicable) who previously received HEI funding should include a brief (up to 300 words) summary of previous HEI-funded studies, including key outcomes, successes, and challenges.
Conflict of Interest
In addition to information on sources of Other Support (Form F-8), HEI requires investigators to disclose any personal conflicts of interest. HEI will treat all disclosures as confidential information, which will be used solely in the application review process and not shared outside HEI. Investigators should report financial relationships with entities in the exposure science or environmental health areas that could influence, or be perceived to influence, the proposed research. Please report all sources of revenue paid (or promised to be paid) directly to you or your institution on your behalf over the past 36 months greater than $3,000. Disclose any personal fees (monies paid to you for services rendered, generally honoraria, royalties, or fees for consulting, lectures, speakers bureaus, expert testimony) and non-financial support (for example, reagents or equipment and travel costs). Report also any patents, whether planned, pending or issued, broadly relevant to the work. Report any other relationships
Letters of Support
A. Consultants. Consultant arrangements and proposed collaborations with investigators at other institutions must be confirmed in writing. Attach appropriate letters from each individual, confirming their role in the project.
B. Other Letters of Support.
Please provide letters of support where applicable, indicating the type of support the collaborators will provide. Examples include
a. Access to facilities
b. Data sharing
c. Collaboration with communities or community groups
Additional Materials (Rosenblith Award only)
Applications to the Walter A. Rosenblith New Investigator Award should include a cover letter appended to the beginning of the application. Please also include a letter indicating institutional support, a mentoring plan with letters from each mentor, three recent publications, and a list of all publications by the candidate to be included as part of Form F-11. Please refer to the RFA for details.
PERSONAL DATA (FORM F-12 — OPTIONAL)
HEI has a continuing commitment to monitoring the operation of its review and award process to detect, and deal appropriately with, real or perceived inequities with respect to age, ethnicity, race, gender, or other personal attributes of the proposed principal investigator. To provide HEI with the information needed to fulfill this commitment, we request that each applicant complete the optional personal data form (Form F-12) and submit it as a separate PDF with the full application. Upon receipt at the HEI office, this form will be separated from the application and used only for internal HEI monitoring procedures. If you do not wish to provide this information, or do not complete the form, it will in no way affect consideration of your application.


